The number of individual side-effects are an important indicator for assessing the risk of taking a so called "Covid-19 vaccine". The European Medicines Agency (EMA) collects individual reports of "suspected adverse reactions" on its database EudraVigilance (www.adrreports.eu).
1. Some media report high numbers of side-effects and deaths
A number of news websites, such as healthimpactnews.com refering to EudraVigilance communicate high numbers of fatal cases:.
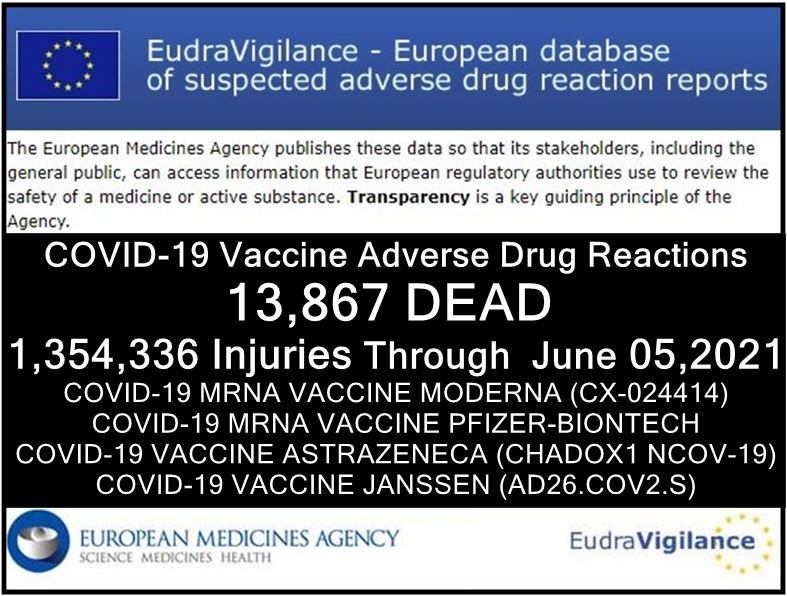
Further below, Brian Shilhavy writes as follows:
Their [i.e. EudraVigilance's] report through June 5, 2021 lists 13,867 deaths and 1,354,336 injuries following injections of four experimental COVID-19 shots:
COVID-19 MRNA VACCINE MODERNA (CX-024414)
COVID-19 MRNA VACCINE PFIZER-BIONTECH
COVID-19 VACCINE ASTRAZENECA (CHADOX1 NCOV-19)
COVID-19 VACCINE JANSSEN (AD26.COV2.S)From the total of injuries recorded, there are 683,688 serious injuries which equals over 50%.
These figures of serious injuries and fatal cases are impressive, but are they correct?
Let's peer review them and build a solid ground for upcoming debates.
2. Fatal cases: 8,727 vs 13,867 of a total of 525,907 vs. 1,354,336
As adrreports.eu does not communicate the sum of fatal cases, you have to dig deeper in order to get out the sum of deaths of suspected side-effects.
I did it and found a much lower figure than Brian Shilhavy and others:
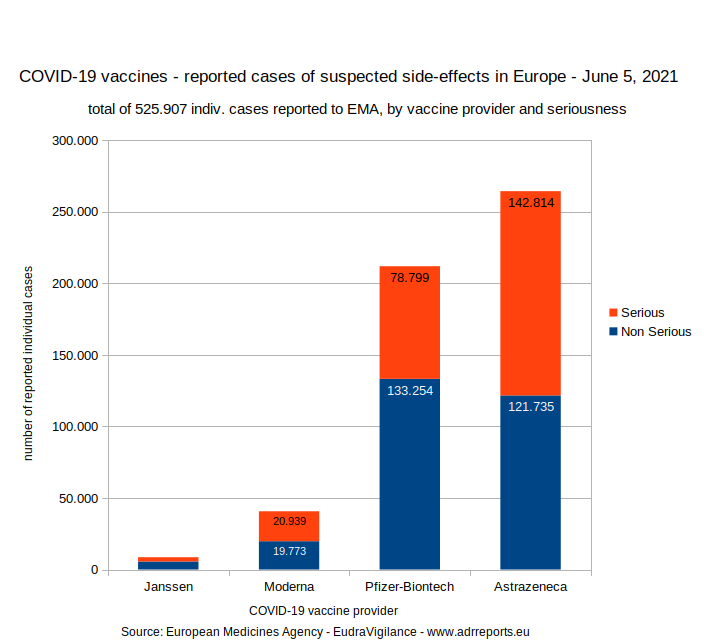
2.1. Total of reported cases with side-effects by seriousness
2.1.1. Chart
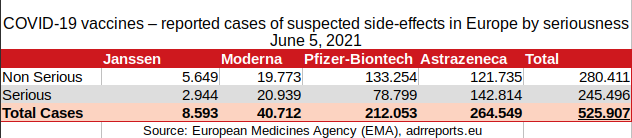
2.1.2. Table
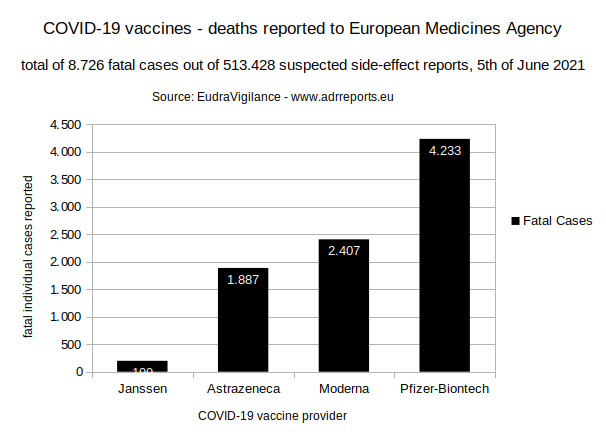
2.2. Total of reported fatal cases
2.2.1. Chart
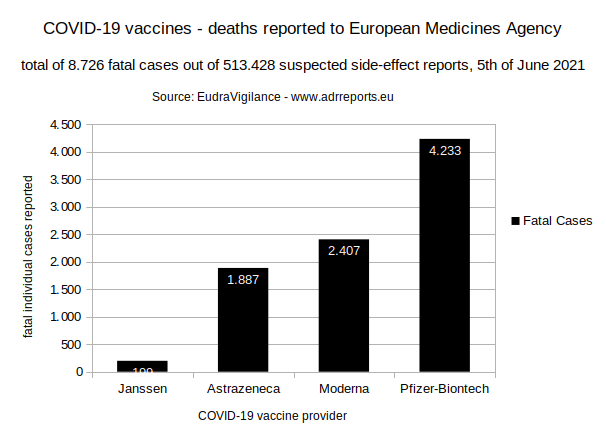
2.2.2. Table
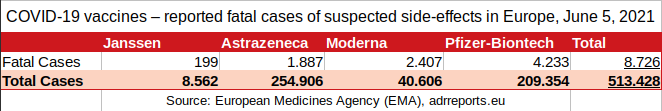
3. How to add EudraVigilance data properly?
3.1. Calculating the total of suspected side-effect cases
It is straightforward to get the total number of reported suspected adverse reactions for all 4 "COVID-19 vaccines" on EudraVigilance. Simply add up the totals of the first tables (see below) showing up on the cover page of each vaccine.
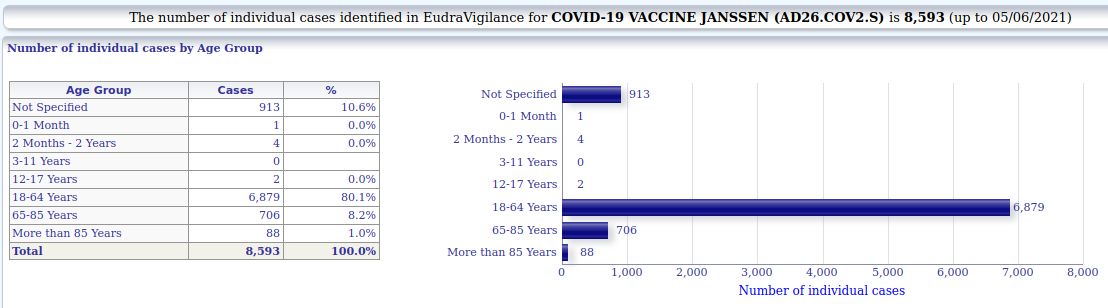
8.593 (Janssen)
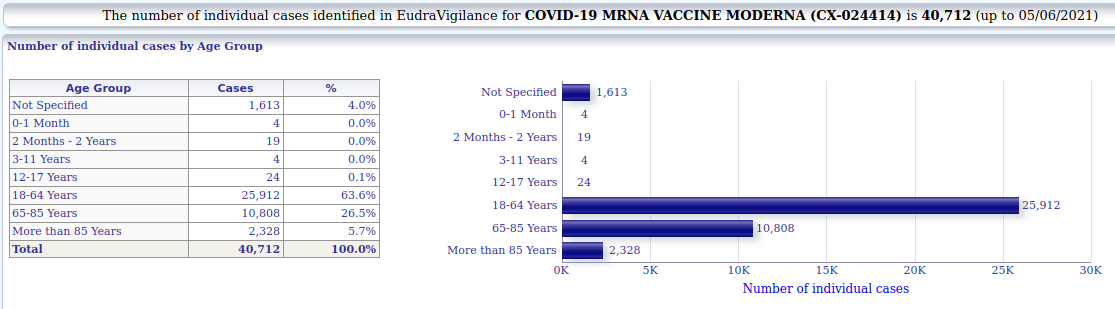
+ 40.712 (Moderna)
+ 212.053 (Pfizer)
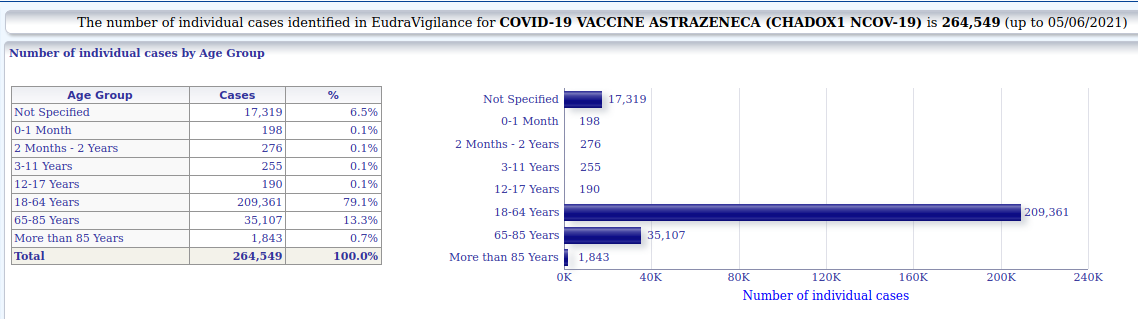
+ 264.549 (Astrazeneca)
====================
525.907 total or reported
====================3.1.1. Janssen - individual cases of suspected side-effects by age
3.1.2. Moderna - individual cases of suspected side-effects by age
3.1.3. Pfizer-Biontech - individual cases of suspected side-effects by age
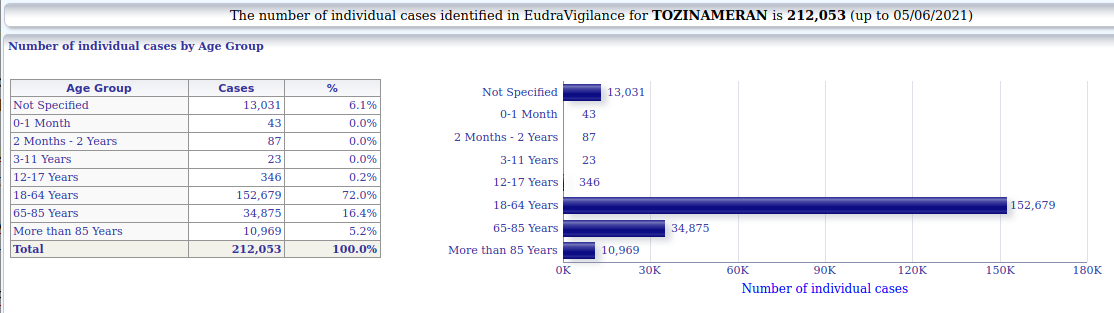
3.1.3. Astrazeneca - individual cases of suspected side-effects by age
3.1.4. How to explain the high number of 1,354,336 "injuries"?
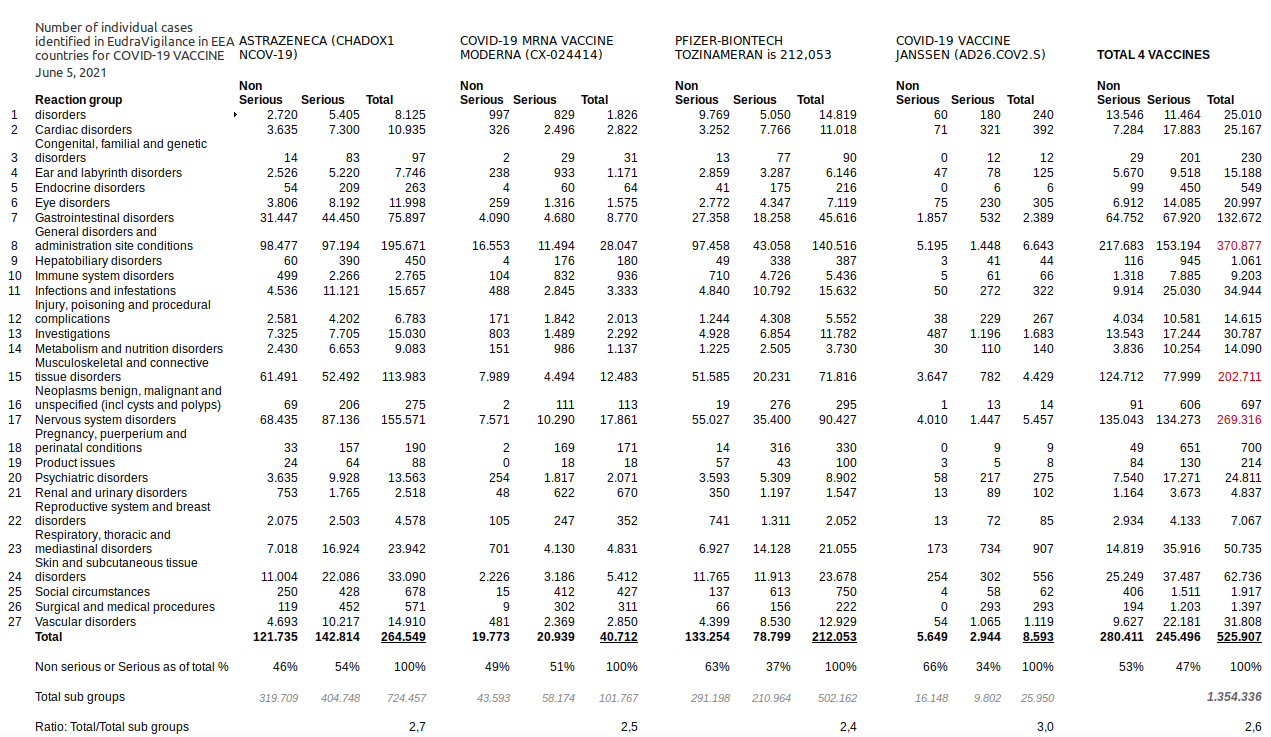
3.1.5. How to explain the wrong calculation of 13,867 deaths?
EudraVigilance reports fatal cases only by reaction group. As each individual reported case is associated to an average of 2,6 reaction groups it is inaccurate to calculate the total number of deaths by adding up the deaths reported for each reaction group because it leads to a double count. Instead you need to parse the individual case reports of EudraVigilance.
That's what we did for each vaccine. Our results are reported above (ref. 2.1.2 Table):
199 (Janssen)
+ 1.887 (Astrazeneca)
+ 2.407 (Moderna)
+ 4.233 (Pfizer/Biontech)
=========================
8.726 total reported fatal cases
=========================
NOTE: This result is based on a total of 513,428 parsed reports only.(1) NOTE
The running total of individual cases available in Tab 1 is the value that should be used to quantify the total number of individual cases that have been reported to EudraVigilance for a selected medicine or active substance.The information available in Tab 3, Tab 4, Tab 5 and Tab 6 takes into account the suspected side effect(s) reported in an individual case; as an individual case; may refer to more than one suspected side effect, the information does NOT represent the total number of individual cases that have been reported to EudraVigilance, but the number of related side effects.
Source: (https://www.adrreports.eu/en/viewing_reports.html)
Disclaimer
Each time you search for a web report on www.adrreports.eu you will be shown a disclaimer. To view individual reports you must confirm that you have read and understood the disclaimer.
The disclaimer contains the following information:
- The information on this website does not reflect any confirmation of a potential link between the medicine and the observed effect(s).
- The information on this website concerns suspected associations that reflect the reporter's observations and opinions. A scientific assessment of a cause-and-effect relationship between a medicine and an effect is part of the continuous monitoring of the benefits and risks of a medicine; the assessment takes into account many other factors, such as the medical condition and the medical history of the patient.
- The information may include known side effects already listed in the summary of product characteristics (SmPC) and the package leaflet.
- The number of suspected side effects in EudraVigilance should not serve as a basis for determining the likelihood of a side effect occurring. This is because the numbers need to be put into context with other factors, such as how many people take the medicine and how long the medicine has been on the market.
- Each individual case in EudraVigilance refers generally to a single patient; however, more than one side effect may have been reported in a report. Therefore, the number of side effects will not always be the same as the number of individual cases.
- The side-effect reports in EudraVigilance do not represent all available information concerning the benefits and risks of a medicine and should not be used in isolation to make decisions regarding a patient's treatment regimen; other sources of information, including the product/prescribing information, should be consulted first.
- Patients and consumers should not stop or change prescribed medication without prior consultation with a healthcare professional.